HSE Scientists Discover Method to Convert CO₂ into Fuel Without Expensive Reagents

Researchers at HSE MIEM, in collaboration with Chinese scientists, have developed a catalyst that efficiently converts CO₂ into formic acid. Thanks to carbon coating, it remains stable in acidic environments and functions with minimal potassium, contrary to previous beliefs that high concentrations were necessary. This could lower the cost of CO₂ processing and simplify its industrial application—eg in producing fuel for environmentally friendly transportation. The study has been published in Nature Communications.
The electrochemical reduction of carbon dioxide is a process in which CO₂ is converted into other chemical compounds through the application of an electric current. It has long been regarded not only as a method for recycling CO₂, but also as a source of valuable raw materials such as formic acid, which can serve as a liquid fuel, solvent, or chemical industry feedstock.
However, a major challenge in the electrochemical reduction of CO₂ is the occurrence of a side reaction that produces hydrogen, thereby reducing the overall efficiency of the process. In alkaline solutions, this issue is typically addressed by adding more potassium ions (K⁺); however, this not only increases the cost of the process but also leads to precipitation, which clogs the system and hinders its operation. Conversely, in an acidic environment, catalysts degrade rapidly and lose their effectiveness.
A team of researchers, including those from HSE University, has proposed an alternative approach. They developed a catalyst that remains stable in acidic environments while requiring only a minimal amount of potassium. The catalyst is made from indium oxide (In₂O₃) and coated with a thin layer of carbon.
First, through computer modelling, the researchers at HSE MIEM determined how to control the distribution of ions on the catalyst's surface. The model revealed that the carbon coating not only protects the catalyst from degradation but also creates an electric field that holds potassium ions on its surface. As a result, potassium does not precipitate, and undesirable side effects are minimised.
To test the model's predictions, Chinese scientists synthesised indium oxide nanoparticles and encapsulated them in a thin layer of carbon. The team then conducted a series of experiments in an electrolyte reactor, using a highly acidic environment and a fraction of the potassium typically used in conventional systems. The tests showed that even under these conditions, the catalyst remained stable, maintaining activity for over 100 hours, with a CO₂ to formic acid conversion efficiency of 98.9%.
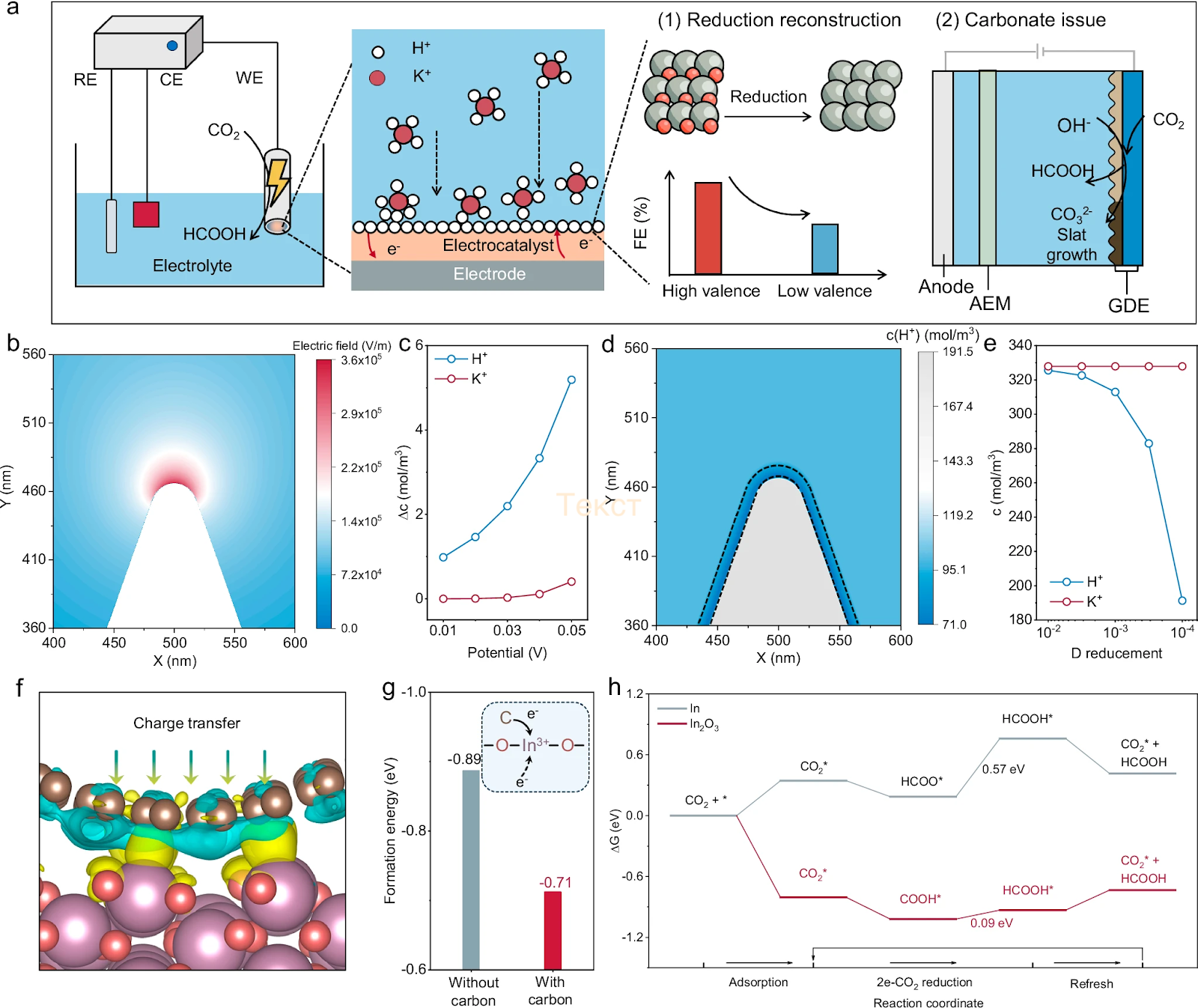
'We have demonstrated that it is possible to eliminate excess potassium hindering the system's operation. This approach not only reduces the cost of the process but also improves catalyst stability,' explains Dongyu Liu, Assistant Professor at HSE MIEM.
To verify that the carbon coating is the key factor, the researchers conducted additional tests. They found that without the coating, indium oxide rapidly reduces to metallic indium, which is far less effective at facilitating the electrochemical reduction of CO₂. This confirms that the carbon layer protects the catalyst, preventing its degradation.
This method not only simplifies CO₂ processing technology but also makes it more accessible for industrial applications. Unlike conventional alkaline systems, it does not require a high concentration of potassium and prevents precipitation. The implementation of this technology in real-world systems could make carbon dioxide recycling more environmentally sustainable.
'We have made the process more stable and scalable, bringing the electrochemical reduction of carbon dioxide closer to real-world industrial applications,' says Andrey Vasenko, Professor at HSE MIEM. 'This technology can be useful not only for synthesising formic acid but also for other processes related to carbon dioxide conversion.'
See also:
Scientists Test Asymmetry Between Matter and Antimatter
An international team, including scientists from HSE University, has collected and analysed data from dozens of experiments on charm mixing—the process in which an unstable charm meson oscillates between its particle and antiparticle states. These oscillations were observed only four times per thousand decays, fully consistent with the predictions of the Standard Model. This indicates that no signs of new physics have yet been detected in these processes, and if unknown particles do exist, they are likely too heavy to be observed with current equipment. The paper has been published in Physical Review D.
HSE Scientists Reveal What Drives Public Trust in Science
Researchers at HSE ISSEK have analysed the level of trust in scientific knowledge in Russian society and the factors shaping attitudes and perceptions. It was found that trust in science depends more on everyday experience, social expectations, and the perceived promises of science than on objective knowledge. The article has been published in Universe of Russia.
Scientists Uncover Why Consumers Are Reluctant to Pay for Sugar-Free Products
Researchers at the HSE Institute for Cognitive Neuroscience have investigated how 'sugar-free' labelling affects consumers’ willingness to pay for such products. It was found that the label has little impact on the products’ appeal due to a trade-off between sweetness and healthiness: on the one hand, the label can deter consumers by implying an inferior taste, while on the other, it signals potential health benefits. The study findings have been published in Frontiers in Nutrition.
HSE Psycholinguists Launch Digital Tool to Spot Dyslexia in Children
Specialists from HSE University's Centre for Language and Brain have introduced LexiMetr, a new digital tool for diagnosing dyslexia in primary school students. This is the first standardised application in Russia that enables fast and reliable assessment of children’s reading skills to identify dyslexia or the risk of developing it. The application is available on the RuStore platform and runs on Android tablets.
Physicists Propose New Mechanism to Enhance Superconductivity with 'Quantum Glue'
A team of researchers, including scientists from HSE MIEM, has demonstrated that defects in a material can enhance, rather than hinder, superconductivity. This occurs through interaction between defective and cleaner regions, which creates a 'quantum glue'—a uniform component that binds distinct superconducting regions into a single network. Calculations confirm that this mechanism could aid in developing superconductors that operate at higher temperatures. The study has been published in Communications Physics.
Neural Network Trained to Predict Crises in Russian Stock Market
Economists from HSE University have developed a neural network model that can predict the onset of a short-term stock market crisis with over 83% accuracy, one day in advance. The model performs well even on complex, imbalanced data and incorporates not only economic indicators but also investor sentiment. The paper by Tamara Teplova, Maksim Fayzulin, and Aleksei Kurkin from the Centre for Financial Research and Data Analytics at the HSE Faculty of Economic Sciences has been published in Socio-Economic Planning Sciences.
Larger Groups of Students Use AI More Effectively in Learning
Researchers at the Institute of Education and the Faculty of Economic Sciences at HSE University have studied what factors determine the success of student group projects when they are completed with the help of artificial intelligence (AI). Their findings suggest that, in addition to the knowledge level of the team members, the size of the group also plays a significant role—the larger it is, the more efficient the process becomes. The study was published in Innovations in Education and Teaching International.
New Models for Studying Diseases: From Petri Dishes to Organs-on-a-Chip
Biologists from HSE University, in collaboration with researchers from the Kulakov National Medical Research Centre for Obstetrics, Gynecology, and Perinatology, have used advanced microfluidic technologies to study preeclampsia—one of the most dangerous pregnancy complications, posing serious risks to the life and health of both mother and child. In a paper published in BioChip Journal, the researchers review modern cellular models—including advanced placenta-on-a-chip technologies—that offer deeper insights into the mechanisms of the disorder and support the development of effective treatments.
Using Two Cryptocurrencies Enhances Volatility Forecasting
Researchers from the HSE Faculty of Economic Sciences have found that Bitcoin price volatility can be effectively predicted using Ethereum, the second-most popular cryptocurrency. Incorporating Ethereum into a predictive model reduces the forecast error to 23%, outperforming neural networks and other complex algorithms. The article has been published in Applied Econometrics.
Administrative Staff Are Crucial to University Efficiency—But Only in Teaching-Oriented Institutions
An international team of researchers, including scholars from HSE University, has analysed how the number of non-academic staff affects a university’s performance. The study found that the outcome depends on the institution’s profile: in research universities, the share of administrative and support staff has no effect on efficiency, whereas in teaching-oriented universities, there is a positive correlation. The findings have been published in Applied Economics.




